Neural Impulse Chip to Make Quadriplegic People Walk Again
Movement Restored: Luke Tynan, who was paralyzed in 2017 past a spinal cord injury, demonstrates the wearable system that enables him to command his arm and hand. Sensors on the arm register his intentions, while electrodes stimulate the nerves and muscles to produce his desired movements.
In 2015, a group of neuroscientists and engineers assembled to watch a man play the video game Guitar Hero. He held the simplified guitar interface gingerly, using the fingers of his right hand to press down on the fret buttons and his left paw to striking the strum bar. What fabricated this mundane scrap of game play so extraordinary was the fact that the man had been paralyzed from the chest down for more than three years, without whatsoever use of his hands. Every time he moved his fingers to play a note, he was playing a song of restored autonomy.
His movements didn't rely on the damaged spinal string inside his body. Instead, he used a technology that nosotros phone call a neural bypass to turn his intentions into actions. First, a encephalon implant picked up neural signals in his motor cortex, which were then rerouted to a computer running machine-learning algorithms that deciphered those signals; finally, electrodes wrapped around his forearm conveyed the instructions to his muscles. He used, essentially, a type of artificial nervous arrangement.
We did that research at the Battelle Memorial Plant in Columbus, Ohio. I've since moved my lab to the Institute of Bioelectronic Medicine at the Feinstein Institutes for Medical Research, in Manhasset, N.Y. Bioelectronic medicine is a relatively new field, in which we apply devices to read and modulate the electrical activity within the body'due south nervous organization, pioneering new treatments for patients. My group'south particular quest is to crack the neural codes related to movement and awareness so we can develop new ways to treat the millions of people around the world who are living with paralysis—5.four million people in the United States lonely. To practise this we start need to sympathize how electrical signals from neurons in the brain chronicle to actions by the body; then we need to "speak" the language correctly and attune the appropriate neural pathways to restore movement and the sense of touch. After working on this problem for more than than 20 years, I feel that we've just begun to understand some key parts of this mysterious code.
 Group Attempt: Study participant Luke Tynan (forepart) works with a squad of researchers to effort out the wearable neural bypass. From left: Republic of chad Bouton, Richard Ramdeo, Santosh Chandrasekaran, and Nikunj Bhagat. Photo: Nathaniel Welch
Group Attempt: Study participant Luke Tynan (forepart) works with a squad of researchers to effort out the wearable neural bypass. From left: Republic of chad Bouton, Richard Ramdeo, Santosh Chandrasekaran, and Nikunj Bhagat. Photo: Nathaniel Welch
My team, which includes electrical engineer Nikunj Bhagat, neuroscientist Santosh Chandrasekaran, and clinical manager Richard Ramdeo, is using that data to build two unlike kinds of synthetic nervous systems. Ane arroyo uses encephalon implants for high-fidelity command of paralyzed limbs. The other employs noninvasive wearable engineering science that provides less precise control, but has the benefit of not requiring brain surgery. That vesture engineering science could also exist rolled out to patients relatively before long.
Ian Burkhart, the participant in the Guitar Hero experiment, was paralyzed in 2010 when he dove into an ocean moving ridge and was pushed headfirst into a sandbar. The impact fractured several vertebrae in his neck and damaged his spinal cord, leaving him paralyzed from the centre of his breast downwards. His injury blocks electric signals generated past his brain from traveling down his nerves to trigger actions by his muscles. During his participation in our study, technology replaced that lost function. His triumphs—which also included swiping a credit card and pouring water from a bottle into a drinking glass—were amidst the outset times a paralyzed person had successfully controlled his own muscles using a brain implant. And they pointed to ii ways forwards for our research.
 Rocking Out: In 2015, report participant Ian Burkhart used the first version of the implant-based neural bypass to play the game Guitar Hero. Photo: Ohio Land University Wexner Medical Center/Batelle
Rocking Out: In 2015, report participant Ian Burkhart used the first version of the implant-based neural bypass to play the game Guitar Hero. Photo: Ohio Land University Wexner Medical Center/Batelle
The system Burkhart used was experimental, and when the study ended, so did his new autonomy. We set out to alter that. In one thrust of our research, we're developing noninvasive wearable technology, which doesn't crave a brain implant and could therefore be adopted past the paralyzed community fairly chop-chop. As I'll draw subsequently in this commodity, tetraplegic people are already using this system to attain out and grasp a variety of objects. We are working to commercialize this noninvasive engineering science and hope to gain clearance from the U.S. Food and Drug Administration within the side by side year. That'southward our short-term goal.
We're also working toward a long-term vision of a bidirectional neural bypass, which will utilize brain implants to choice up the signals close to the source and to return feedback from sensors nosotros'll place on the limb. We promise this two-style system will restore both motion and sensation, and we've embarked on a clinical trial to test this arroyo. We desire people like Burkhart to experience the guitar as they make music with their paralyzed hands.
Paralysis used to be considered a permanent status. But in the by ii decades, there'due south been remarkable progress in reading neural signals from the brain and using electric stimulation to power paralyzed muscles.
In the early on 2000s, the BrainGate consortium began groundbreaking piece of work with brain implants that picked upwards signals from the motor region of the brain, using those signals to control various machines. I had the privilege of working with the consortium during the early years and developed automobile-learning algorithms to decipher the neural lawmaking. In 2007, those algorithms helped a woman who was paralyzed due to a stroke bulldoze a wheelchair with her thoughts. By 2012, the team had enabled a paralyzed woman to utilize a robotic arm to pick upwards a canteen. Meanwhile, other researchers were using implanted electrodes to stimulate the spinal cord, enabling people with paralyzed legs to stand up upward and even walk.
My research grouping has continued to tackle both sides of the problem: reading the signals from the brain likewise equally stimulating the muscles, with a focus on the hands. Around the time that I was working with the BrainGate squad, I remember seeing a survey that asked people with spinal cord injuries about their top priorities. Tetraplegics—that is, people with paralysis of all four limbs—responded that their highest priority was regaining part in their arms and hands.
Robotics has partially filled that need. One commercially available robotic arm tin can be operated with wheelchair controls, and studies have explored controlling robotic arms through brain implants or scalp electrodes. But some people still long to employ their own arms. When Burkhart spoke to the press in 2016, he said that he'd rather not take a robotic arm mounted on his wheelchair, considering he felt it would describe besides much attention. Unobtrusive technology to control his ain arm would allow him "to role nearly as a normal member of society," he said, "and not be treated as a cyborg."
Restoring movement in the hands is a daunting challenge. The human hand has more than 20 degrees of freedom, or ways in which information technology can move and rotate—that's many more than the leg. That means there are many more muscles to stimulate, which creates a highly complex control-systems problem. And nosotros don't yet completely sympathize how all of the hand's intricate movements are encoded in the brain. Despite these challenges, my group fix out to give tetraplegics dorsum their hands.
Burkhart'south implant was in his brain'south motor cortex, in a region that controls hand movements. Researchers take extensively mapped the motor cortex, so there's plenty of data near how general neuronal activity at that place correlates with movements of the paw as a whole, and each finger individually. But the corporeality of information coming off the implant's 96 electrodes was formidable: Each ane measured activity 30,000 times per second. In this torrent of data, we had to find the discrete signals that meant "flex the thumb" or "extend the index finger."
To decode the signals, we used a combination of bogus intelligence and man perseverance. Our stalwart volunteer attended up to iii sessions weekly for 15 weeks to train the system. In each session, Burkhart would watch an blithe hand on a computer screen motion and flex its fingers, and he'd imagine making the aforementioned movements while the implant recorded his neurons' action. Over time, a auto-learning algorithm figured out which pattern of activeness corresponded to the flexing of a thumb, the extension of an index finger, and and so on.
Once our neural-bypass system understood the signals, it could generate a pattern of electric pulses for the muscles of Burkhart'south forearm, in theory mimicking the pulses that the brain would send down an undamaged spinal cord and through the nerves. But in reality, translating Burkhart's intentions to muscle movements required another intense round of preparation and calibration. We spent endless hours stimulating different sets of the 130 electrodes wrapped around his forearm to make up one's mind how to control the muscles of his wrist, hand, and each finger. Simply we couldn't duplicate all of the movements the hand tin can make, and we never quite got control of the little finger! We knew we had to develop something better.
Grab a Snack: Casey Ellin, who was partially paralyzed past a spinal cord injury, tests out an before epitome version of the wearable neural bypass arrangement. Video: The Feinstein Institutes for Medical Research
To make a more applied and convenient organisation, we decided to develop a version that is completely noninvasive, which nosotros call GlidePath. We recruited volunteers who have spinal cord injuries just nonetheless have some mobility in their shoulders. We placed a proprietary mix of inertial and biometric sensors on the volunteers' arms, and asked them to imagine reaching for different objects. The information from the sensors fed into a machine-learning algorithm, enabling the states to infer the volunteers' grasping intentions. Flexible electrodes on their forearms then stimulated their muscles in a item sequence. In ane session, volunteer Casey Ellin used this wear bypass to choice up a granola bar from a tabular array and bring it to his oral cavity to take a bite. We published these results in 2020 in the journal Bioelectronic Medicine.
My squad is working to integrate the sensors and the stimulators into lightweight and inconspicuous wearables; nosotros're besides developing an app that will exist paired with the vesture, so that clinicians can check and conform the stimulation settings. This setup will allow for remote rehabilitation sessions, because the information from the app will be uploaded to the deject.
To speed up the process of calibrating the stimulation patterns, we're building a database of how the patterns map to hand movements, with the help of both able-bodied and paralyzed volunteers. While each person responds differently to stimulation, there are enough similarities to train our organisation. It's analogous to Amazon'due south Alexa voice assistant, which is trained on thousands of voices and comes out of the box ready to get—but which over time further refines its understanding of its specific users' speech patterns. Our wearables volition likewise be ready to become immediately, offer bones functions like opening and closing the hand. Just they'll go on to learn about their users' intentions over fourth dimension, helping with the movements that are about important to each user.
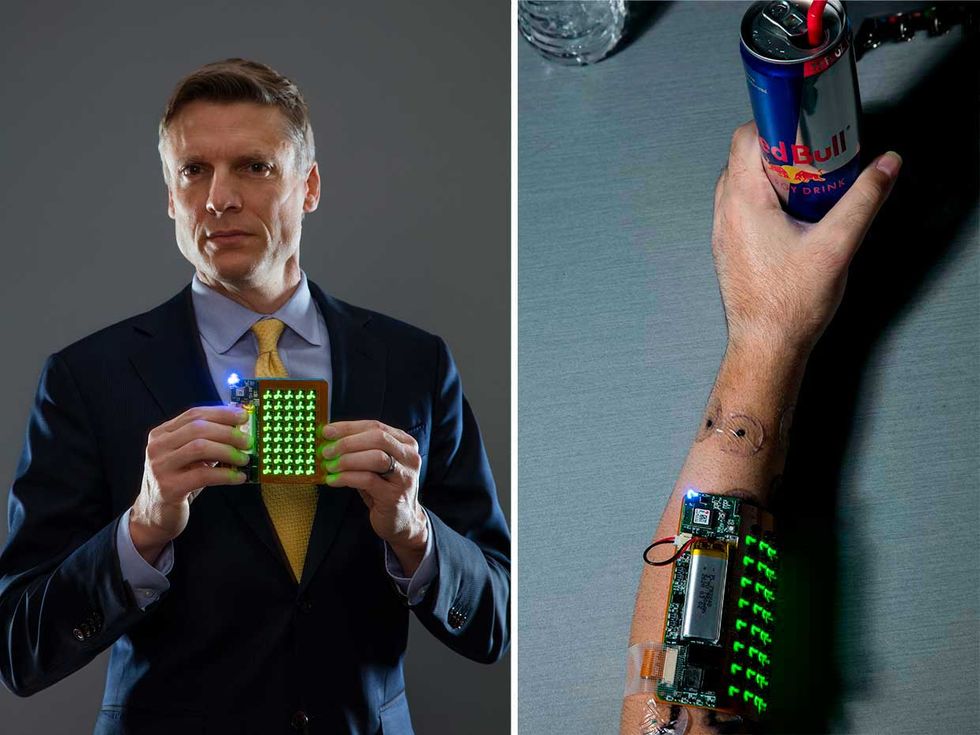 Patching Through: Chad Bouton (left) holds the latest version of the wearable patch that stimulates nerves and muscles when placed on the user's forearm (correct). Photos: Nathaniel Welch
Patching Through: Chad Bouton (left) holds the latest version of the wearable patch that stimulates nerves and muscles when placed on the user's forearm (correct). Photos: Nathaniel Welch
Nosotros think this technology tin can help people with spinal cord injuries also as people recovering from strokes, and we're collaborating with Practiced Shepherd Rehabilitation Hospital and the Barrow Neurological Establish to test our technology. Stroke patients commonly receive neuromuscular electric stimulation, to assist with voluntary movements and help recover motor role. At that place'due south considerable show that such rehab works improve when a patient actively tries to make a movement while electrodes stimulate the proper muscles; that continued effort by brain and muscles has been shown to increase "plasticity," or the ability of the nervous system to arrange to damage. Our system volition ensure that the patient is fully engaged, as the stimulation will be triggered by the patient'due south intention. We plan to collect information over fourth dimension, and we hope to meet patients eventually regain some function fifty-fifty when the engineering is turned off.
Equally heady as the article of clothing applications are, today'due south noninvasive engineering science doesn't readily control complex finger movements, at least initially. Nosotros don't wait the GlidePath engineering science to immediately enable people to play Guitar Hero, much less a real guitar. So we've continued to work on a neural bypass that involves brain implants.
When Burkhart used the earlier version of the neural bypass, he told us that it offered a huge step toward independence. Merely in that location were a lot of practical things we hadn't considered. He told us, "Information technology is strange to not feel the object I'1000 holding." Daily tasks like buttoning a shirt require such sensory feedback. Nosotros decided so to work on a 2-style neural featherbed, which conveyed movement commands from the encephalon to the hand and sent sensory feedback from the hand to the brain, skipping over the damaged spinal cord in both directions.
To give people sensation from their paralyzed easily, we knew that we'd demand both finely tuned sensors on the hand and an implant in the sensory cortex region of the brain. For the sensors, we started by thinking near how human skin sends feedback to the brain. When you pick something upwards—say, a disposable cup filled with coffee—the pressure compresses the underlying layers of skin. Your peel moves, stretches, and deforms every bit you lift the cup. The thin-moving-picture show sensors we adult can discover the pressure level of the cup against the skin, as well as the shear (transverse) force exerted on the peel every bit yous lift the cup and gravity pulls it down. This frail feedback is crucial, considering there'south a very narrow range of advisable movement in that circumstance; if you squeeze the cup too tightly, you'll end up with hot coffee all over yous.
Each of our sensors has different zones that detect the slightest pressure or shear strength. Past aggregating the measurements, our organization determines exactly how the pare is bending or stretching. The processor will send that data to the implants in the sensory cortex, enabling a user to experience the cup in their hand and suit their grip as needed.
 Touch and Experience: An fMRI image (top) shows brain activity associated with hand movements. The two-way bypass records from the motor cortex and stimulates the sensory cortex. Thin-film sensors (bottom) measure pressure level and forcefulness; that information goes to stimulating electrodes in the sensory cortex. Photos, height: The Feinstein Institutes for Medical Research; bottom: Abigail Bouton
Touch and Experience: An fMRI image (top) shows brain activity associated with hand movements. The two-way bypass records from the motor cortex and stimulates the sensory cortex. Thin-film sensors (bottom) measure pressure level and forcefulness; that information goes to stimulating electrodes in the sensory cortex. Photos, height: The Feinstein Institutes for Medical Research; bottom: Abigail Bouton
Figuring out exactly where to stimulate the sensory cortex was another challenge. The part of the sensory cortex that receives input from the hand hasn't been mapped exhaustively via electrodes, in office because the regions dealing with the fingertips are tucked into a groove in the brain called the central sulcus. To fill in this bare spot on the map, we worked with our neurosurgeon colleagues Ashesh Mehta and Stephan Bickel, along with hospitalized epilepsy patients who underwent procedures to map their seizure activeness. Depth electrodes were used to stimulate areas inside that groove, and patients were asked where they felt sensation. We were able to elicit sensation in very specific parts of the manus, including the crucial fingertips.
That knowledge prepared us for the clinical trial that marks the next step in our research. We're currently enrolling volunteers with tetraplegia for the study, in which the neurosurgeons on our team volition implant iii arrays of electrodes in the sensory cortex and ii in the motor cortex. Stimulating the sensory cortex volition likely bring new challenges for the decoding algorithms that interpret the neural signals in the motor cortex, which is correct side by side door to the sensory cortex—at that place will certainly be some changes to the electric signals we pick up, and we'll have to learn to compensate for them.
In the study, we've added 1 other twist. In addition to stimulating the forearm muscles and the sensory cortex, we're also going to stimulate the spinal cord. Our reasoning is as follows: In the spinal cord, in that location are 10 million neurons in complex networks. Earlier research has shown that these neurons have some power to temporarily directly the torso's movements fifty-fifty in the absence of commands from the brain. We'll have our volunteers concentrate on an intended move, to physically make the motility with the aid of electrodes on the forearm, and receive feedback from the sensors on the hand. If we stimulate the spinal cord while this process is going on, nosotros believe we tin promote plasticity within its networks, strengthening connections between neurons inside the spinal cord that are involved in the hand's movements. It's possible that we'll achieve a restorative event that lasts beyond the duration of the study: Our dream is to requite people with damaged spinal cords their hands back.
One day, we promise that brain implants for people with paralysis will be clinically proven and approved for employ, enabling them to go well beyond playing Guitar Hero. Nosotros'd similar to see them making complex movements with their easily, such as tying their shoes, typing on a keyboard, and playing scales on a pianoforte. We aim to allow these people attain out to clasp easily with their loved ones and experience their touch in return. Nosotros desire to restore movement, sensation, and ultimately their independence.
This article appears in the February 2021 print issue as "Bypassing Paralysis."
Source: https://spectrum.ieee.org/brain-implants-and-wearables-let-paralyzed-people-move-again
0 Response to "Neural Impulse Chip to Make Quadriplegic People Walk Again"
Post a Comment